Health care
“Silent Murder”
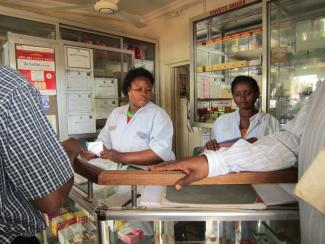
According to the World Health Organisation (WHO), “essential medicines” are those that satisfy the priority health needs of the people. The WHO list of such medicines currently includes 340 drugs. The problem, however, is that these pharmaceuticals are not available to all people who need them.
According to Daisy Isa from Nigeria, 60 % of essential medicines are not accessible to people in Africa, South-East Asia and the Western Pacific. Isa works for CHAN Medi-Pharm, an ecumenical drug supply organisation in Nigeria. She lists two main reasons for the shortfalls:
- Often only medicines like analgesics are manufactured in developing countries, while remedies for life-threatening diseases like TB or HIV/AIDS are imported, and therefore much more expensive.
- Supply-chain management tends to be poor in developing countries, which results in shortages and product deterioration, for instance, when vaccines are stored too hot or too cold.
Storage and distribution problems, of course, are linked to poor infrastructure in general.
Deadly medicine
As people with average incomes in developing countries often cannot afford quality drugs, they turn to cheaper offers – counterfeit medicines for example. Doing so is dangerous, however, because the medications are likely to be ineffective for healing purposes or even poisonous. Hiiti Sillo, the director general of the Tanzania Food and Drugs Authority, puts it bluntly: “Counterfeit medicines are silent murder.”
Counterfeit drugs are often packaged just like brand medicines, so it is very difficult for pharmacists to tell the difference. As Sillo points out, there are not enough control laboratories in many African countries. Laws on medicine tend to be inadequate, and penal sanctions are often quite weak. Smuggling of pharmaceuticals is endemic. Sillo says that “combating counterfeit medicines requires collaboration at national, regional and international level”.
Local production of generic drugs could make the difference. Generic drugs are chemically the same as brand drugs, but they are not produced by multinational giants. They are a lot cheaper than brand products, but just as good.
Once a pharma patent has expired, generic drug production is permitted everywhere. According to the rules of the World Trade Organisation (WTO), moreover, pharma patents to do not apply to least developed countries before 2016. Another WTO rule authorises all national governments to allow companies to produce generic versions of patent-protected medicines if doing so is necessary for public-health reasons.
The German civil-society organisation action medeor provides emergency and disaster relief, but also helps to improve medical supply in developing countries. At a conference action medeor held in Bonn in February, its experts pointed out that generic pharma manufacturers in developing countries face several challenges, including:
- lack of qualified personnel, in particular pharmaceutical technicians,
- lack of basic infrastructure and utilities that would ensure reliable water and power supply,
- lack of government regulations concerning medical quality control,
- limited access to active pharmaceutical ingredients (APIs – the ingredients of drugs that make them effective),
- limited access to other relevant inputs such as excipients (substances used to carry APIs in medicines) and reagents (chemicals needed to test the quality of a drug),
- lack of funds and poor access to credit, as well as
- lack of good distribution networks.
Once generic pharma production takes off, however, the drugs supply in developing countries tends to improve fast. Due to their strong generics industries, India and Brazil are now exporting affordable generic versions to many developing countries.
For all countries that wish to copy their example, staff training is a core challenge. For the time being, says Eliangiringa Kaale of Mhuimbili University’s School of Pharmacy in Dar es Salaam, there are only two options: “We can either import skilled personnel, for instance from India, or – if a lab cannot afford to do so – take the risk to manufacture medicines without adequate attention to quality standards.” Pharmaceutical laboratories in Africa often resort to the latter, which is detrimental to the health of the people concerned.
In spite of the challenges, action medeor supports local generics production, provided that quality control is ensured. “Local producers have a long-term interest in developing and keeping local markets”, says Bernd Pastors, the chief executive of the organisation.
Germany’s Federal Ministry for Economic Cooperation and Development (BMZ) and its implementing agencies similarly assist local enterprises in producing high quality essential medicines. The idea is to create an “excellence hub” in East Africa, says Frank Schmiedchen of BMZ. He emphasises that his ministry is not only interested in the transfer of know-how and technology, but just as much in improving the regulatory environment.
The WHO is similarly in favour of a double agenda. It too wants to speed up technology transfer for generics production on the one hand, and to contribute to improving pharma governance on the other.
“Poor people deserve good quality medicines,” says Lembit Rägo of the WHO. He emphasises that quality drugs are not only needed in the fight against malaria and other tropical diseases. They are needed to treat illnesses of all kinds. According to him, 70 % of patients with mental disorders in low-income countries do not have access to adequate medicines. Producing inexpensive drugs is urgently needed – for body and mind.
Sheila Mysorekar